General chemistry
PFAS stands for per- and polyfluoroalkyl substances. As PFAS do not naturally occur in the environment, they need to be synthesised industrially. They are manufactured from hydrocarbons such as petroleum.
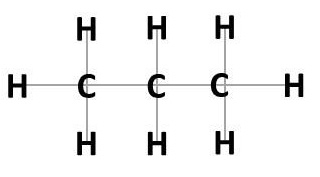
Hydrocarbons are chemicals that are composed of a chain of carbon atoms (C) of varying length with two or three hydrogen atoms bound to each carbon (three hydrogen atoms bound to the carbon atom at the end of the chain, and two to the carbon atoms mid- chain) (Figure 1).
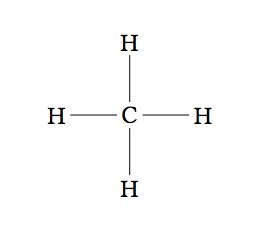
The simplest hydrocarbon is methane with only one carbon atom in the middle that binds to four hydrogen atoms surrounding it (Figure 2).
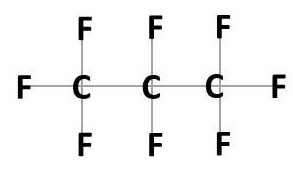
In the manufacturing process, the hydrogen atoms are fully or partially replaced with fluorine atoms (F). As the bond between carbon and fluorine is extremely strong, the process requires a large amount of energy. The powerful C-F bonds are the main cause for the persistence of PFAS in the environment. If the hydrogen atoms are fully replaced with fluorine, the resulting substances are called perfluoroalkyl such as Perfluorobutanoic acid (Figure 3). If the hydrogen atoms are only partially replaced, they are called polyfluoroalkyl.
The number of different per- and polyfluoroalkyl substances (PFAS) ranges between 5,000 to 10,000, and not even all existing PFAS are known and described. Specific groupings of atoms give rise to characteristic properties of the classes of organic compounds, these classes are called functional groups. Familiar examples of functional groups include alcohols such as ethanol, ketones such as acetone and carboxylic acids such as acetic acid. The names and chemical qualities of each PFAS are mostly determined by the functional groups that are attached to the main part of the PFAS molecule.
Perfluoroalkyl substances
In perfluoroalkyl substances all hydrogen atoms are replaced with fluorine. Below are the most common types of perfluoroalkyl substances that are determined by their functional group.
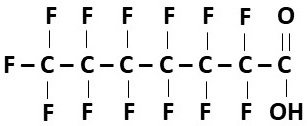
- Perfluoroalkyl carboxylic acids (PFCAs):
- Functional group: Carboxylic acid (-COOH)
- Example: Perfluoroheptanoic acid (PFHpA) (Figure 4)
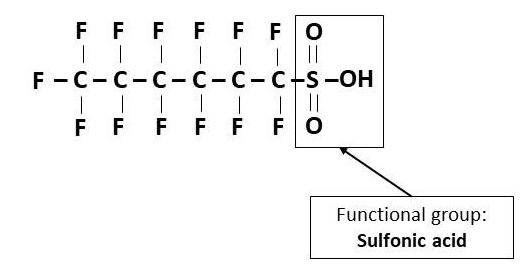
- Perfluoroalkane sulfonic acids (PFSAs)
- Functional group: Sulfonic acid (- SO3H)
- Example: Perfluorohexane sulfonic acid (PFHxS) (Figure 5)
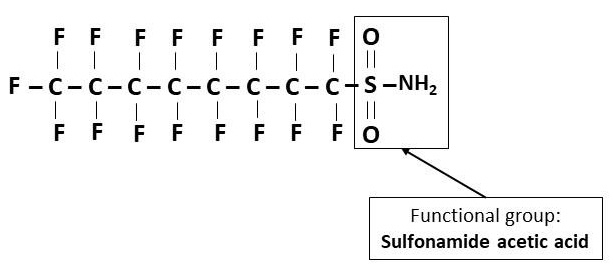
- Perfluoroalkane sulfonamides (FASAs)
- Functional group: Sulfonamide acetic acid (- SO2NH2)
- Example: Perfluorooctane sulfonamide acetic acid (FOSA/PFOSA) (Figure 6)
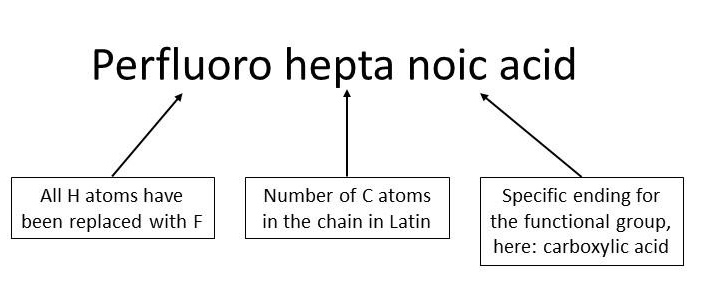
General nomenclature (simplified) of perfluoroalkyl substances
The general nomenclature is explained below, on Perfluoroheptanoic acid (PFHpA) as an example.
Polyfluoroalkyl substances
In polyfluoroalkyl substances only some hydrogen atoms are replaced with fluorine. Polyfluoroalkyl substances can also carry any of the above-mentioned functional groups (carboxylic acid (-COOH), sulfonic acid (- SO3H) or sulfonamide acetic acid (- SO2NH2)). However, their chemical formulas are commonly more complex, carrying multiple functional groups. Polyfluoroalkyl substances include many of the more modern PFAS like ADONA (4,8-dioxa-3H-perfluorononanoic acid), shown in Figure 8, and are used as replacements to some of the older long-chain PFAS types such as PFOS and PFOA.
Fluorinated polymers
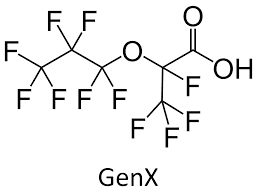
These are even more complex substances consisting of a carbon or oxygen backbone with fluorine (F) attached to the carbon (C) atoms. Like the polyfluoroalkyl substances, fluorinated polymers include many of the more modern PFAS like GenX (Figure 9) and are used as replacement for some of the older long-chain PFAS, such as PFOS and PFOA.